Table of contents
An increasing awareness of preventive health measures has created a larger demand for a variety of health food supplements in Indonesia, leading to opportunities for manufacturers to enter health food supplement sector in the country.
Health supplement registration in Indonesia is regulated by the National Agency for Drug and Food (BPOM) that claims the health food supplement market in Indonesia have made around USD313 million from imported products. Currently, United States is the main exporter for health food supplement market in Indonesia, accounting for about 70% of the market.
This article provides you with a guidance for establishing a business that imports and distributes food supplements in Indonesia.
Registration for Imported Food Supplements
In Indonesia, health food supplement is regulated through Regulation of The Head of The National Drug and Food Control Agency Republic of Indonesia (BPOM) No. HK.00.05.41.1381 concerning The Standard Procedure of Food Supplement Registration.
Based on the regulation, if you wish to import food supplement, your company must be in:
- A pharmaceutical industry, or
- A traditional drug industry, or
- A food industry, or
- A business entity in food supplement marketing sector that receives appointment letter directly for the industry in the country origin.
It is also important for you to note that :
- Without having Indonesian entity (Company) as above, it is not possible to register your products in Indonesia.
- You must have a Company in Indonesia/ Appoint an Indonesian company to be your registrar (INDOSIGHT has service to act as their registrar to, in the event they do not want to open Company in Indonesia).
- Your company is also obliged to possess importer license in pharmaceutical preparation sector and for this, Emerhub can provide you an undername import service if you want to import without holding the license.
- To comply with the requirements and standards for food supplement imports into Indonesia, your company that produces food supplement in your country origin is obliged to meet requirements of Good Manufacturing Procedures (GMP) proven with certification issued by the competent government authority or an accredited certification institution and, if needed, close inspection can be done by Head of The National Drug and Food Control Agency (BPOM) officer.
Notably, all imported health food supplements may only be distributed through a local agent or distributor, such as Emerhub and must be registered with the BPOM before clearance through the Customs.
The health food registration process takes around 3 to 6 months once the required documents are complete. The documents you submitted to us must be clear and true as misleading information about your products or company is forbidden and is subject to criminal proceedings if found by the BPOM.
Data Safety and Approval of Registration
Many clients are concerned about their patents and details of ingredients, as those are very sensitive issues. However, you don’t need to worry about it anymore as the safety of your company products data is guaranteed and will be kept confidential by the Agency Head, in accordance with Article 9 of the Regulation of The Head of National Agency of Drug and Food Control (BPOM) No. HK.00.05.41.1381 on Procedure of Food Supplement Registration.
Validity
The registration number is valid for (5) years
Compliance After the Registration Numbers are Issued:
- Products should be distributed no longer than (1) year after the issuance of the registration number
- The packaging design should be submitted at least (1) month before importation
- The import realization report should be submitted to The Head of The National Drug and Food Control Agency every (6) months
Food Supplement Registration Category
Food supplement registration is categorized into new registration and variation registration as shown in table below.
| Registration Category | |
| New Registration | Variation Registration |
|
Category 1 Registration of food supplement that contains one or more materials in the form of :
|
Category 4 Registration of food supplement that has obtained circulation license with:
|
|
Category 2 Registration of food supplement that contains one or more materials in the form of:
|
Category 5 Registration of food supplement that has obtained circulation license with:
|
|
Category 3 Registration of food supplements of categories 1 and 2 with a claim of:
|
Category 6 Registration of food supplement that has obtained circulation license with:
|
Health Food Supplement Registration Process
Registration of food supplement is conducted in 2 stages, namely pre- assessment and assessment as seen in below.
To avoid delay in assessing your application you are encouraged to first consult with us regarding the required documentation. Our aim is to make sure that you meet the standards that are relevant to your registration and to understand our guidance.
We will work with you during this time and keep you informed of progress, and advise what steps you need to take to expedite the approval process.
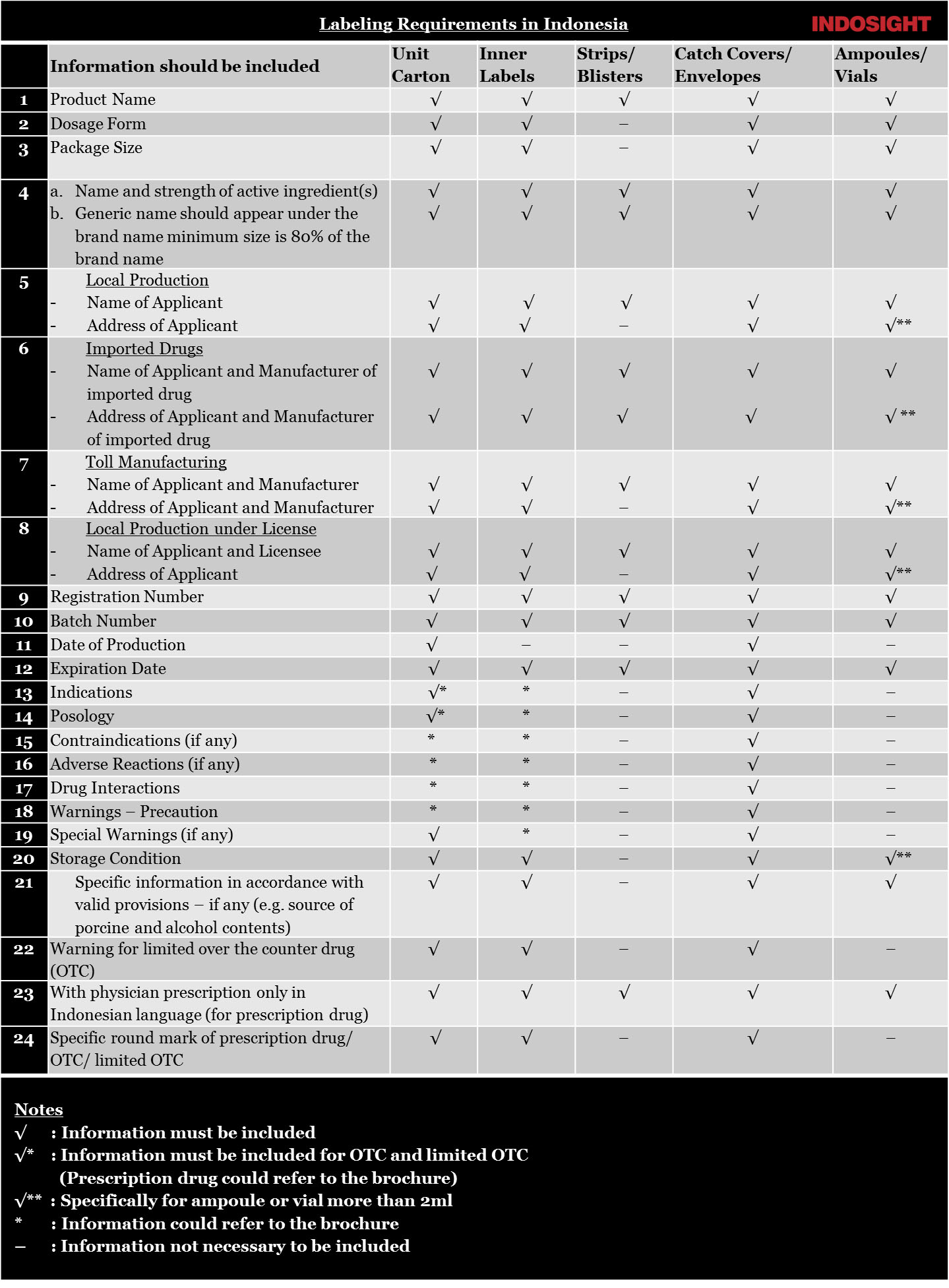
Labeling Design Minimum Requirement
You are responsible for developing labels (including nutrition information) that meet legal food labeling requirements. All labeling of BPOM- regulated food products must be truthful and not misleading. Proper labeling, including nutrition labeling and labeling for the major food allergens, is required for most prepared foods.
The labels of food products sold in Indonesia interstate commerce must be in Indonesian.
Market for Health Food Supplement Products in Indonesia
With increasingly busy lifestyles, Indonesian consumers are becoming more aware of the positive impact of nutritional products. The market for health supplements has enjoyed phenomenal growth at an annual rate of around 22% from 2014 to 2015. Imports of health food supplements are expected to increase over 23% in the end of 2016.
The awareness of the positive effects of health food supplements on health has supported the sales for these products, thus increasing the demand for health food supplements registration in Indonesia.
Sales Prospects
The best sales prospects for Indonesia are:
- Diet and aesthetics products – items that enhance physical appearance, including weight loss
- Degenerative prevention products – items that prevent degenerative diseases such as cardiovascular diseases, hypertension and osteoporosis
- Products that improve stamina and immunity
- Vitamins
Prospective Buyers
Upscale consumers who belong to the higher income group have the ability to buy food supplements of more than IDR1,400,000/ USD100 per month.
Middle to upper- class consumers have the ability to buy an average of IDR700,000/ USD50 worth of health food supplement products per month, whereas the rest are estimated to be consumers who spend less than IDR140,000/ USD10 per month.
Conclusion
Changes in recent years have seen a reduction of the Government’s controls on food imports and distribution, but imports are still highly regulated. The most difficult problem for exporters shipping high value food supplement products may be the requirement that all imported products be registered. This can be a long and onerous process, but Emerhub can get it all done.
Here are the services Emerhub provides related to food supplement registration in Indonesia:
- Carrying out an initial consultation into how The Head of National Agency of Drug and Food Control’s (BPOM’s) regulations on health food supplements apply to your registration
- Guiding you through the pre- assessment (i.e., Administrative, Technical and Supporting documents) and assessment process
- Handle the compliance post registration (i.e., the import realization report that should be submitted to BPOM every 6 months)
- Provide an undername import service if you did not have an import license in Indonesia
- Keeping you updated with your application and giving you the most services until the registration process is complete
Emerhub is a market entry consultancy with a focus on providing legal consultancy services in addition to market entry or market expansion and business advisory services.
Therefore, after your good supplement registration’s completed , our consultants who have worked with a multiple of well- known agencies could give you a consultation in how to produce successful products that are of good quality, well- packaged, well- distributed, well- promoted and competitively priced in the market.
Contact us today for more information about entering health food supplement sector in Indonesia.